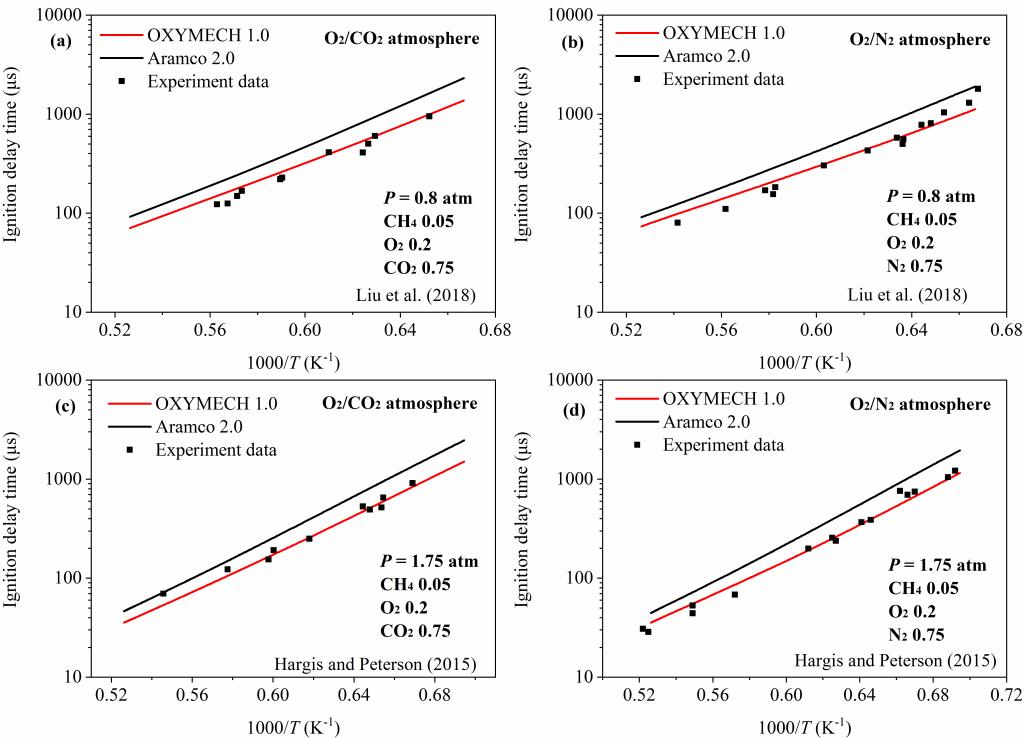
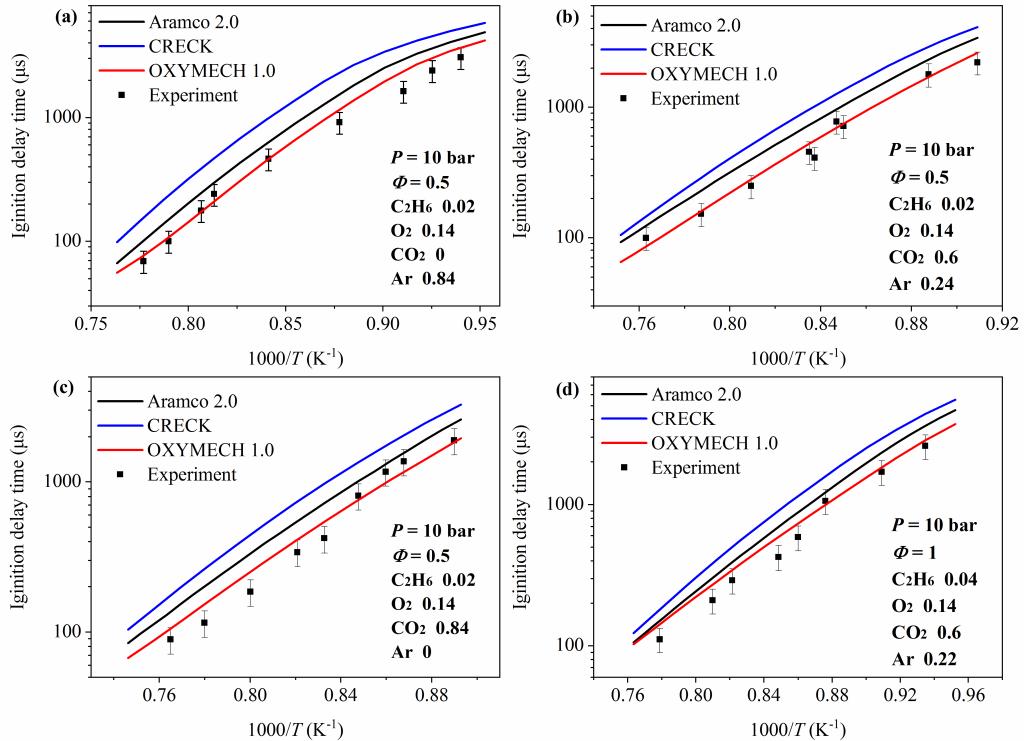
本课题组已经发展了适用C0-C3富氧气氛和空气气氛的详细反应动力学模型OXYMECH。在Aramco 1.3的基础上对其中一些基元反应的反应速率常数进行了更新修正,如表1所示,使修改后的模型能够更好的与现有的富氧气氛下甲烷的着火延迟时间相匹配,并在此基础上进一步进行更新与优化得到预测乙烷着火延迟时间的模型OXYMECH 1.0,其主要更新、优化的化学反应和相应的化学反应速率常数见表2。
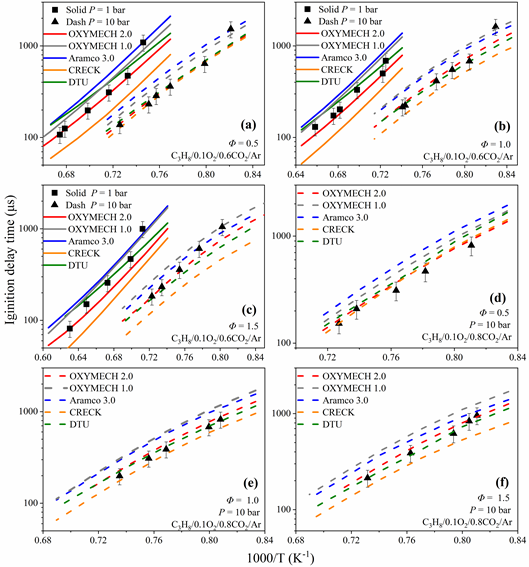
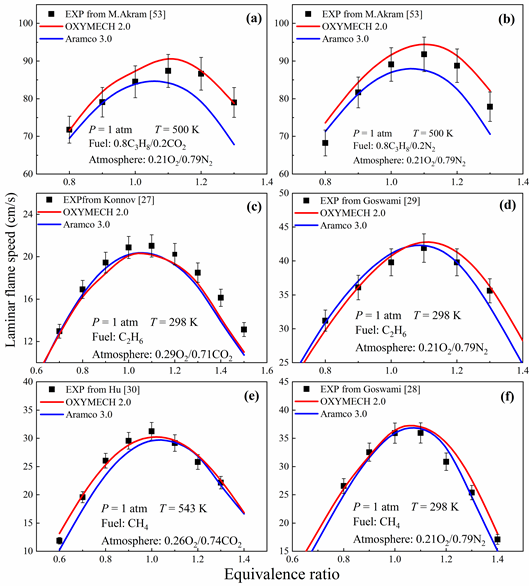
对OXYMECH 1.0的C3子机理中基元反应速率常数进行优化,使其能准确预测丙烷在富氧气氛下的着火延迟,同时对影响火焰传播速度的基元反应速率常数进行优化,从而得到能准确预测C0-C3富氧气氛和空气气氛下着火延迟时间与火焰传播速度的模型OXYMECH 2.0,其主要更新、优化的化学反应和相应的化学反应速率常数见表3。
参考文献:
1. Y. Liu, C. Zou, J. Cheng, H. Jia, C. Zheng, Experimental and Numerical Study of the Effect of CO2 on the Ignition Delay Times of Methane under Different Pressures and Temperatures, Energy & Fuels 32 (2018) 10999-11009.
2. Y. Liu, J. Cheng, C. Zou, L. Lu, H. Jing, Ignition delay times of ethane under O2/CO2 atmosphere at different pressures by shock tube and simulation methods, Combust. Flame 204 (2019) 380-390.
表1 甲烷富氧燃烧机理中更新的反应
Reaction |
A |
n |
EA |
CH4 + OH ó CH3 +H2O |
2.30E+08 |
1.40 |
2850.0 |
CH4 + Hó CH3 + H2 |
4.78E+05 |
2.50 |
9588.0 |
CH3 + CH3 (+M) ó C2H6 (+M) |
2.50E+13 |
0.00 |
0.00 |
Low-pressure limit
|
2.33E+34 |
-5.03 |
-1200.0 |
a = 0.38, T3 = 73.0, T1 = 1180.0 |
表2 乙烷富氧燃烧机理中更新的反应
Reaction |
A |
n |
EA |
C2H6 + HO2 ó C2H5 + H2O2 |
1.10E+05 |
2.5 |
16850 |
C2H6 + OH ó C2H5 + H2O |
9.46E+06 |
2.0 |
994 |
C2H6 + H ó C2H5 + H2 |
1.13E+08 |
1.9 |
7530 |
C2H6 + CH3 ó C2H5 + CH4 |
5.60E+10 |
0.0 |
9420 |
Duplicate |
C2H6 + CH3 ó C2H5 + CH4 |
8.30E+14 |
0.0 |
22260 |
Duplicate |
C2H6 + O ó OH + C2H5 |
1.76E+05 |
2.8 |
5803 |
C2H5 + O2 ó HO2 + C2H4 |
1.36E+07 |
1.1 |
-1975 |
C2H4 + H (+M) ó C2H5 (+M) |
1.23E+09 |
1.5 |
1355 |
Low-pressure limit |
2.03E+39 |
-6.6 |
5769 |
a = 1.6, T3 = -9147, T1 = 299,T2 = 152 |
C2H4 + OH ó C2H3+H2O |
2.14E+04 |
2.7 |
2216 |
C2H4 + H ó C2H3 + H2 |
2.20E+02 |
3.6 |
11270 |
2CH3 ó H + C2H5 |
7.62E+12 |
0.1 |
1060 |
CH3 + HO2 ó OH + CH3O |
8.82E+12 |
0.0 |
-590 |
CH3 + HO2 ó O2 + CH4 |
1.27E+05 |
2.2 |
-3022 |
CH3 + O ó H + CH2O |
5.72E+13 |
0.0 |
0 |
HCO + H ó H2 + CO |
8.48E+13 |
0.0 |
0 |
CO + OH ó H + CO2 |
6.19E+04 |
2.1 |
-356 |
Duplicate |
CO + OH ó H + CO2 |
5.0E+12 |
-0.7 |
332 |
Duplicate |
H2O2 (+M) ó 2OH (+M) |
2.19E+12 |
0.9 |
48750 |
Low-pressure limit |
2.49E+24 |
-2.3 |
48750 |
a = 0.58, T3 = 30, T1 = 90000 ,T2 = 90000 |
H+O2 ó O + OH |
9.84E+13 |
0.0 |
15310 |
2HO2 ó H2O2 + O2 |
1.96E+11 |
0.0 |
-1409 |
Duplicate |
2HO2 ó H2O2 + O2 |
1.11E+14 |
0.0 |
11040 |
Duplicate |
HO2 + H ó H2 + O2 |
2.95E+06 |
2.1 |
-1455 |
HO2 + H ó 2OH |
5.89E+13 |
0.0 |
300 |
HO2 + H ó O + H2O |
1.63E+12 |
0.0 |
0 |
OH + H2 ó H + H2O |
2.26E+08 |
1.5 |
3437 |
H + O2 (+M) ó HO2 (+M) |
4.57E+12 |
0.4 |
0 |
Low-pressure limit |
6.37E+20 |
-1.7 |
525 |
a = 0.5, T3 = 30, T1 = 90000 ,T2 = 90000 |
表3 OXYMECH 2.0中更新的反应
Reaction |
A |
n |
EA |
C3H8 + H <=> iC3H7 + H2
|
3.2E+13 |
0 |
7,900 |
C3H8 + OH <=> iC3H7 + H2O
|
1.81E+05 |
2.437 |
-536 |
C3H8 + H <=> nC3H7 + H2
|
1.27E+14 |
0 |
10,500 |
C3H6 <=> C3H5-A + H
|
1.08E+71 |
-15.91 |
124,860 |
Duplicate |
6.28E+42 |
-8.51 |
98,004 |
C2H3 + O2 <=> CH2CHO + O
|
3.00E+11 |
0.29 |
11 |
HO2 + O <=> OH + O2
|
4.00E+13 |
0 |
0 |
HCO + H <=> H2 + CO
|
5.00E+13 |
0 |
0 |
CH + H <=> C + H2
|
7.90E+13 |
0 |
160 |
CH + H2 <=> H + CH2
|
1.75E+14 |
0 |
-165 |
CH3 + O <=> H + CH2O
|
8.43E+13 |
0 |
0 |
CH3 + OH (+M) <=> CH3OH (+M)
|
6.30E+13 |
0 |
0 |
CH2OH (+M) <=> H + CH2O (+M)
|
5.40E+11 |
0.454 |
3600 |
HCCO + H <=> CH2(S) + CO
|
1.00E+14 |
0 |
0 |
C2H2 + O <=> H + HCCO
|
1.63E+07 |
2 |
1900 |
C2H2 + OH <=> H + CH2CO
|
2.18E-04 |
4.5 |
-1000 |
CH2CHO + O <=> H + CH2 + CO2
|
1.50E+14 |
0 |
0 |
C2H4 + OH <=> C2H3 + H2O
|
2.23E+04 |
2.745 |
2216 |
模型的验证如下图:
机理下载:
1. OXYMECH 1.0.
2. OXYMECH 2.0.